Warning: arning: Graphic content, readers’ discretion advised. This story contains a recollection of crime and can be triggering to some readers discretion advised.
On Monday 13 March 2006, eight healthy young men took part in a clinical trial of an experimental drug known as TGN1412. The drug was intended to treat leukaemia and had already been successfully tested on monkeys but never on humans.
It should have been a routine clinical trial but it soon spiralled into one of the most infamous medical emergencies in recent British history.

In 2006 eight men signed up to test a new drug.
When byou take a new drug into humans for the first time,it’s never without risk….
It hd been successfully tested on monkeys-but never on humans.
Doctors faced a medical mystery.
The outcome would change how medical trials are conducted throughout Europe…
But how and why did it happen?
The Srug Trial Emergency at the Hospital

Parexel is a large multinational contract research organization,they had a site which they leased from Northwick Park Hospital and they were doing clinical trials independently from the hospital on early phase development of new drugs.
In February 2006, eight men apply to take part in three day clinical trial of a new experimental drug.
TGN1412 is a type of drug called a monoclonal antibody, it’s important to realize ,that westand on the threshold of a revolution in the way certain types of Illnesses , particularly cancers are treated.
The traditional aproach to treating cancers has been options such as surgery ,chemotherapy or radiotherapy.
Chemotherapy agents as you probably know are essentially poisons, so TGN1412 was intended to treat cancers by educating our own immune system into dealing with them, but that’s clearly a preferable option.
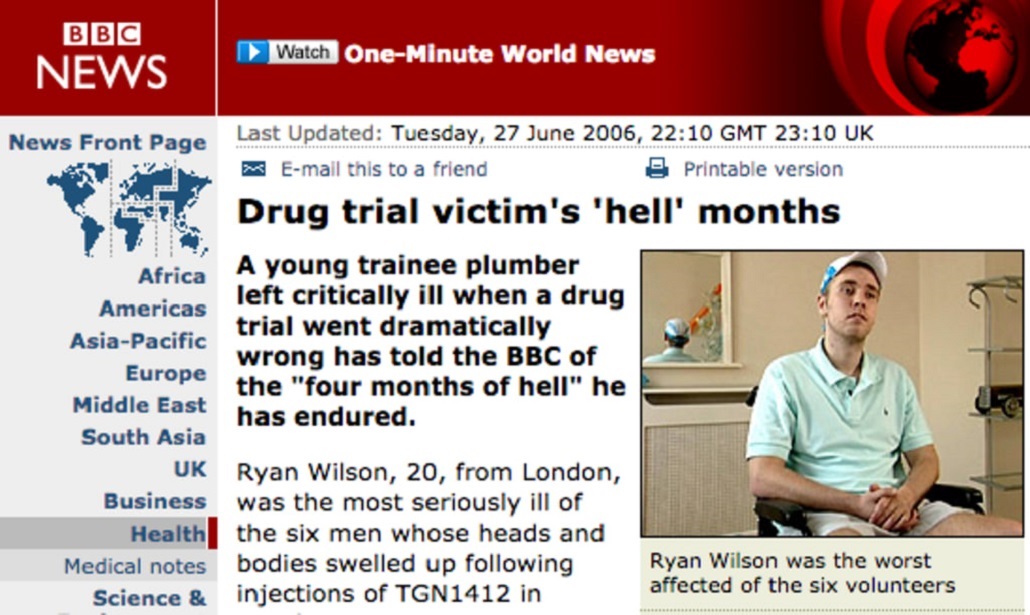
Every one of the drugs that we all commonly use were once first used in humans,they have to go through that stage before they go into bigger trials and then into widespread use.
This was a drug that had shown great promise in animal studies and TGN1412 trial was a first in man study and the first study in humans is about getting to understand how the body deals with the medicine,how it handles it.

It’s important to know that when one participates in a first in man’s study all that’s possible should have been done in the preclinical studies to limit any anticipated risk.
But when you take a new drug into humans for the first time it’s never without some risk.
Monday 13th march 2006 . TGN1412 trial days.

The trial was a double -blind randomized controlled trial and that means that some of the men would have had the active treatment and two of them will have had a placebo or dummy treatment that has no effect.
Neither the clinicians nor the men themselves would know who has the active or inactive treatment.
Just to get a good idea of how the body handles the drugs then it’s better to have healty people usually men because there’s always the risk to reproductive system of study women.

The drug company looked ast the highest dose without any adverse effects in monkeys and then they scaled that back 500 fold to give the first dose in humans giving what they felt was subtantial leeway in terms of safety.

O8:OO the trial begins , david , patient 001 , is first to receive the infusion.
Parexel’s doctor incharge is Daniel Bradford.

Dr Breadford’s written testimony -The Guardian 2016
As a medical student i volunteered for lots of drug trials to make extra money, after graduated i worked as a junior doctor in hospitals before joining the private drugs company that ran trials , i had been involved in more than 300 trials when i was put in charge of testing a new drug.
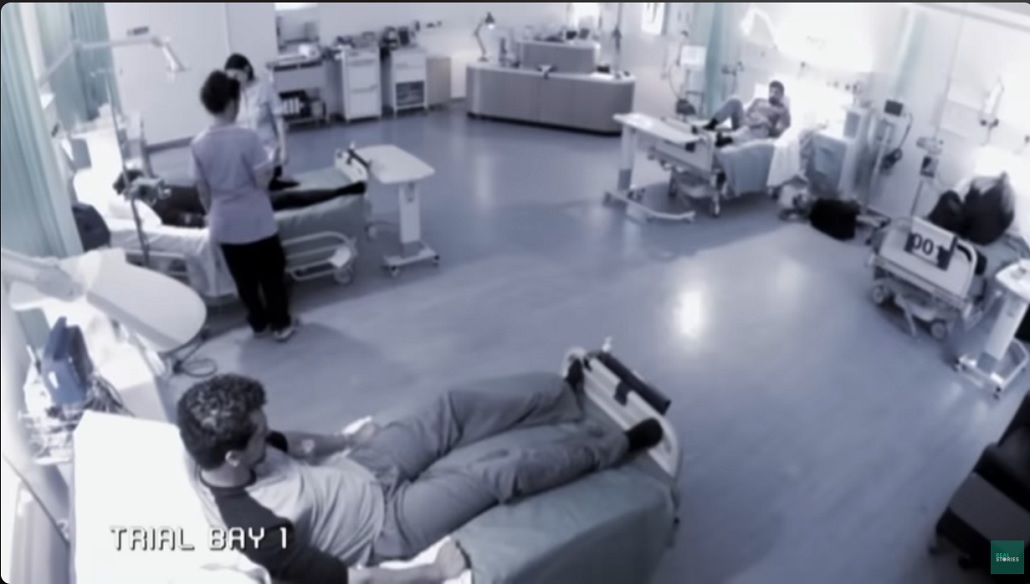
O8:10 ten minutes since trial began, patient 002,is next to receive the infusion.
O8:40 forty minutes since trial began,patient 005 ,receives the infusion.
O8:50 fifty minutes since trail began
09:20,eighty minutes sine trial began – all men have now been infused with TGN1412 or the placebo.

Then they tumbled like dominoes….It was all manic,everything was happening all at once,they were womiting, they were screaming in pain, people fainting,they couldn’t control their bowels.
for some reason patient three started to think he would do better if he got out of this place,he was saying things like ” i dont want the money anymore i just want to get out ,he’s just startd panicking , started freaking out.
He was begging , when he was saying he wanted to go home,he screaming in pain which was the most kind of heorin kind of moments because he was in complete agony, he just freaked ,he just thought that they were out to get him and that he would do better if he actually got out of the hospital as if getting out of the hospital the pain will go away.
The wards became chaotic the men were getting worse,their bodys was in shock,there was chaos, the nurses would take him off guard.
the doctors not knowing what to do,they were probably afraid ,they probably hoped it would only last a short time, they weren’t expecting it to carry on and get worse and everything else that happened.

12:00 four hours since trial began.
It was like a horror movie with the way it was set up as time was progressing…
This must have just been terrible for everyone involved,clearly it was catastrophic for the volunteers but it would have been really scary for the medical staff as well, it’s not something you expect to see in your lifetime doing clinical trials
And suddenly there are people who getting extremely ill all around you,it was obvious that there was something really serious going on.

I couldn’t reach the medical registrar,i ended up running down two flights of stairs to the intensive care unit and grabbing the first two doctors i saw.
The NHS doctors advised the parexel team to administer fluids , antihistamine and treatment dose of Hydrocortisone.

13 :00 five hours since trial began , the two patients who received the placebo are sent home.

one minute you’re in a wards with patients who sounded like thy fighting for their life and then you come back and they’re gone.
What’s going on , what’s happening?
The remaining six patients have now been moved into one trial bay so parexel can monitor them as a group.
19:00 eleven hours since trial began.
and then it seem to be a problem with another patient, all of sudden some surgeon guys came up and they threw the curtain around him.
They then wheeled him away, connected to these machines.
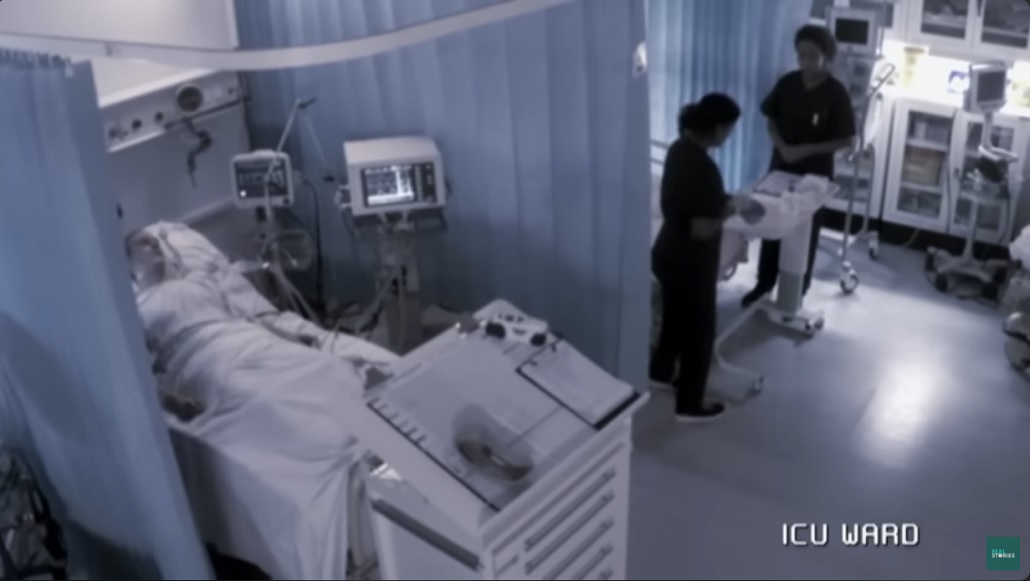
A drug no human had before an unknown illness there was six of them one of them in intensive care,things were changing very quickly .
20:00 twelve hours since trial began.
Blood pressure was very law and th esettings on the ventilatior showed that his lungs have been quite badly affected ,they were having to set things to a fairly high level to keep him safe and stable.
It was clear this was a very serious situation,they also knew there were five more people up there who had received the same drug.
Clock was ticking mthe rate at which they were deteriorating was very rapid, the doctors from the hospital was seriously concerned.
This was in the hands of the NHS now and they obviously have to have got different criteria ,they’re in it for saving lives not for making money as well.

The men all had multiple orgqan failure,someone said it looked as though one man might die and Dr Breadford might be charged with manslaughter. he felt completely out of control.
They were giving in to kidney failure ,they had a f full intensive care unit in that stage,they generated five extra beds in the recovery area,what they didn’t have was enough kidney machine to suddenly provide extra support.
So by this stage of the night there were police vans criss- crossing carrying equipment from many hospitals all converging to Northwick Park

This was a major incident by now and things were pretty complex there were a lot of unknowns,there’s no rulebook for how to deal with his .
Looking at them from the outside they had unstable blood pressures ,their breathing was affected they were developing organ failures,they looked like people who had become septic.
This happens when you have blood poisoning from infection.
They looked as though, they might have an infection so one of the possibilities was that the infusions were contaminated in in some way.
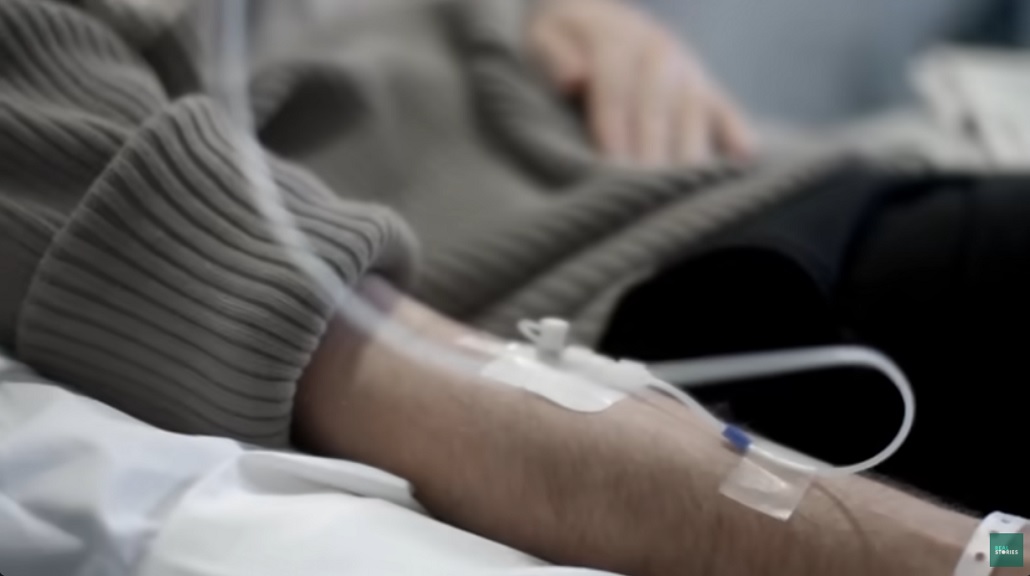
They all received injections from a batch of vials so , allthough it would have been made under steril conditions there always a risk that something would have been happened.

Daniel bradfors kept going back to read the document that detailed everything known about the drugs,it mentioned the unlikely possibility of a cytokine storm an extreme immune reaction.
Cytokines are small proteins involved in the inflammatory response either to an injury or to an external infection .
Cytokine storm is when these become super active throughout the body, leads to a cascade of activity, cells attacking other cells immune cells attacking a tissue ,and that can lead to organ failure,inflammation ,high temperatures, a very nasty outcome.
The clinical trials team told cytokine releases syndrom is a possible effect profile for this drug,the treatment for that would be give really powerfull immune suppressant drugs .
High doses of steroids, the trouble was if the doctors did that and actually this was infection,if some reason they were all reacting to a severe infection then doctors could have made them worse rather then better.
This was so incredulous to have six healty volunteers in a first- in-man trial with such a drug, all at once being very unwell, this is unprecedented.
From the sings and symptoms that was describing,it was more consistent with cytokine release syndrome by that point in time and it should be treated as so and aggressibly so.
But if this was infection then it would have the opposite effect it would damped down the immune system,six people who were fighting with an overwhelming infection .
Patients could deteriorate to the point of death,these patients may die,so there’s really no margin for error.
It was a big decision to take but it’s one doctor’s had to get on with because even the drugs they were giving for this would take time to work,so,doctor’s made the decision,they would treat them as cytokine release syndrome.

so a thousan miligram of steroids is a significant dose, all the doctors could do is wait and see.
what you seeing is his response to that kind of pain that they tried to deal with , a patient was in very serious condition he looked different that what they had been given was starting to swell their bodies.
he just wouldn’t look quite the same.
His cheeks were very swollen,so much so that his eyes ,they look like more slits,his face was just round like a ball and his stomack was huge.


The news caught wind of this story partly because that women said
” they look like Elelphant man”
So everybody wanted to get a glimpse of these atrocious monsters.
In the morning the story was all over the front pages ,somebody had used the words Elephant man after seeing the swollen face and body of one of the patients, it became a headline that stuck.
There were people appearing on camera speculating what this might be what might be going on.
When anyone’s admitted to intensive care and as they start to receive all them support,the treatment that doctors give them can make them look even more distressing, they can be a shock the fluid that’s been given them can make them swollen because you’re giving fluid in and it’s leaking out .
The cause of it there’s really not the clinical trial.
Authorities say is virtually unprecedented in British medical history.

The media interest although very understandable did add pressure ,there were comments that this is very serious ,that they could die.
This left doctors in a very difficult situation it’s unknown territory treating a reaction to an untested drug .
The patient had by now two doses of steroids but doctors coldn’t tell if the things they were doing we’re going to help in the long run.
You give the treatment that you think is best,in this case everythingn was deteriorating further.
Scotland Yard has also become involved it’s tryint to establish if there was any foul play at all, if any of the drugs had been tampered with in any way .

09 :00 tuesday 14th march ,the police contact the govenrnment body that regulates clinical trials- The MHRA.
On the Tuesday morning there was a phone call from the special crime unit in the police,something had gone very much wrong was a clinical trial .
A tragedy a crisis affected the lives of at least six young .

This was treated as acrime scene,the policr seized not only the drug that had been administer but also the placebo ,they seized clinical samples ,blood samples, something could have been tampered with , sabotage,poisoned and that these folk might have been the victims of such foul play.

These drugs were seized and held under the rules of criminal evidence so they came in sealed evidence bags.
The first invasion level is that somebody made a mistake,it was the wrong drug,it mislabeled, contaminated with something,it was the wrong strenght.
The basic pharmaceutical science of this drug had not been carried out properly and of course included in that list of possibilities is a possibility that someone had deliberately adulterated this drug.
These are real people, what had gone wrong, this needed to be investigated.
While doctors trying to save the lives of six volunteersother experts were already trying to find out why they all suffered such extreme reactions what should have been a routine.
Underlying it all ,this was still a mystery, no one knew if this was going to get more severe or if this was the extent of it.
The first round of tests that were carried out of identity and purity tests were able to show very quickly using the simple chemical test that this was the right drug,it was the right strength and it wasn’t contaminated with anything else that would have caused this reaction,the next step was to undestand why tyhose preclinical safety tests hadn’t worked.
Wednesday 15th march-08:00 forty eight hours since trial began.
Six men remain in intensive care , after falling dangerously ill while taking part in clinical trials .
suddenly on wednesday morning on a ward round ,something’s happened the four patinet who were awake suddenly told ,almost within a hour of each other” i feel much better .”

after two days or so that’s when David Oakley felt things were improving.
his life wasn’t on the line anymore.
Every one seemed to perk up,some feeling really hungry and feeling that they was gonna be safe,the fever went down at about the same time and even the two patients who are already on ventilators were in multi-organ failure needing a lot of support their fevers went down some of their numbers started to getting better,the signs of the underlying information seemed to be burning out .
Everyone felt relief but also ,doctors had to ensure that they weren’t going to miss something n feeling that relief.
It was a long way from knowing that they were completely out of the woods but it was the first sign doctor’s had that things might be turning a corner and it was striking how it all happened in all simultaneously.
It was almost like a switch going off.

The men were taken off the machines , the doctors felt that their organs were back to a state which they could start doing their job again .
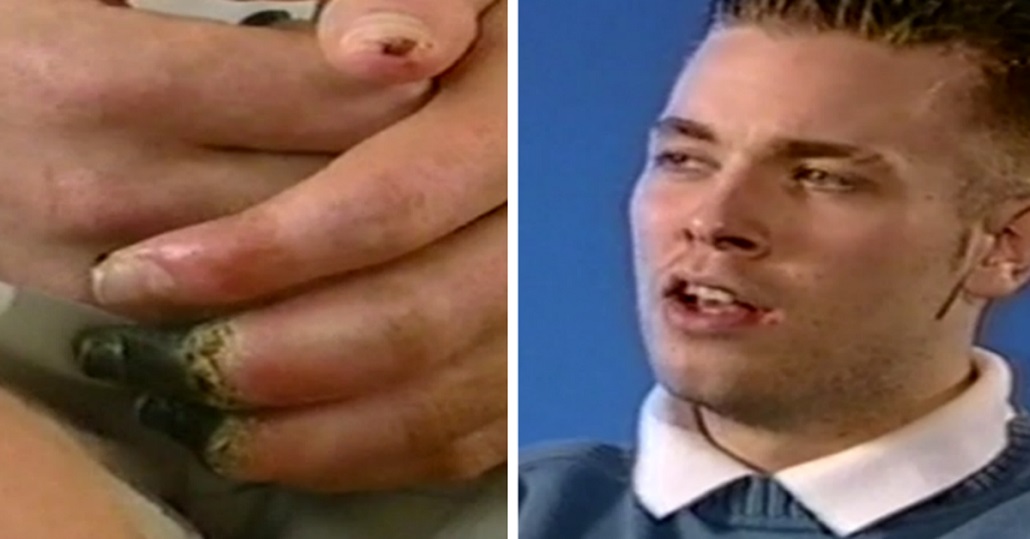
His fingers have blackened and there was no hope for saving the fingers.
He was certainly in a worse condition and he’d come off of this in very bad way and someone was responsible for that.
Ryan was in hospital for four months , he suffered pneumonia, septicaemia and dry gangrene.
His fingertips fell off and h e had part of his foot and toes amputated.
One of the most shocking features off the drug trial was the spacing between the doses.patients were being injected with this new monoclonal antibody in quite quick succession.
Some of the patients were left actually starting to experience problems whilst others were actually been injected for the first time that even from a layman’s point of view seems ridiculous, frankly.
It’s another significant concern,they seem to receive the whole drug in three to six minutes, im not sure that the preclinical testing on animals was done at that pace.
In the human trial , the drug was administered ten times quicker into the bloodstream than it had been to the monkeys.

Those findings were that the trial had been conducted as intended and MHRA – laboratories NIBSC had not discovered anything that was deliberately or unintentionally wrong with the way in which that trial had been managed.
The victims, ,felt the MHRA report was a report that was rushed out and unfortunately was a bit of a whitewash.

The MHRA saying that it was unpreditable,is just their way of passing blame because they’re the ones who said it was an okay trial to do.
The descisions that were taken were entirely appropriate and consistent with the current state of scientific knowledge and MHRA don’t think anybody can be held responsible for knowing something that they don’t know and they couldn’t know at the time.
Per exhale embarked upon a medical trial and they were ill-prepared they didn’t have any backup.
Every thing they did had followed protocol,if you knew what was going to happen,you wouldn’t do it,that’s why you do trials
/Dr bradford.
They should have hd an idea of what to do,think positive things didn’t really start happening to treat us until they involved the outside community and they had the answers/David Oakley

There were people saying thatesd rushed and they didn’t feel that it was happening in a … considerate way ,it was all about being turned over on with the next one ,on with the next one
And so we felt probably disgruntled like we were just cogs in the machine and we were human guinea pigs and nobody wants to fell that way /Rob oldfield , patient 007.

But scientists still couldn’t explain why TGN1412 acted the way it did….
they had no scientific or rational explanation,it was dangerus in humans and completely ineffective in the monkeys.
The biggest problem with that study was that three of the pre-clinical tests gave an answer which suggested there wasn’t a risk ,whether actually was.

Over the next three to four years they came to understand why,they know of course the macaques and humans are very similar species, the t-cells which is the particular cell of the immun system that this drug is supposed to target have on their surface a molecular structure called a receptor which binds to the drug and when the drug binds to the receptor the cell is stimulated to respond.
They also know that the monkey t-cell receptor and the human reset are virtually identical but despite the fact that the monkey t-cell binds TGN1412 it has a similar receptor to the humans it does not respond to the TGN1412 stimulus in the way that the human t-cell does and it does not stimulate this kind of t-cell proliferation an cytokine response in monekys without an additional stimulus.
One might call in a clinical trials community as a never event,it should never happen and that’s why this has changed how first in human studies are done internationally.

The independent review was commissioned by the secretary of state for health,the findings of the review are really very interesting in that they recognized and developed areas where we could improve our practices.
One of the critical recommendations was that where individuals are receiving a drug for the first time,that drug shouldn’t be given to a number of volunteers all in the same day,there were other recommendations in relation to the size of the doze that individuals should receive.
Clinical trials are certainly safer than they have ever been,because there is now more attention to the potential risks.

The raport has been adopted by the European medical agency and therefore applied to the whole of Europe,so those are recommendations that have had a long and hopefully long -lasting effect.
That’s was the last first- in man trial i did, as doctors we swear to do not harm, i felt guilty every day for years,i’d like to look them in the eye and say sorry.”
/Dr Bradford’swrittn testimony, 2016


They used 0.1% of the antibody that was used in London Trial and it was infused into the blood stream at least forty times more slowly.
This type of monoclonal antibody could stillbe used as an effectie form of treatment for auto- immune disease in the future…
